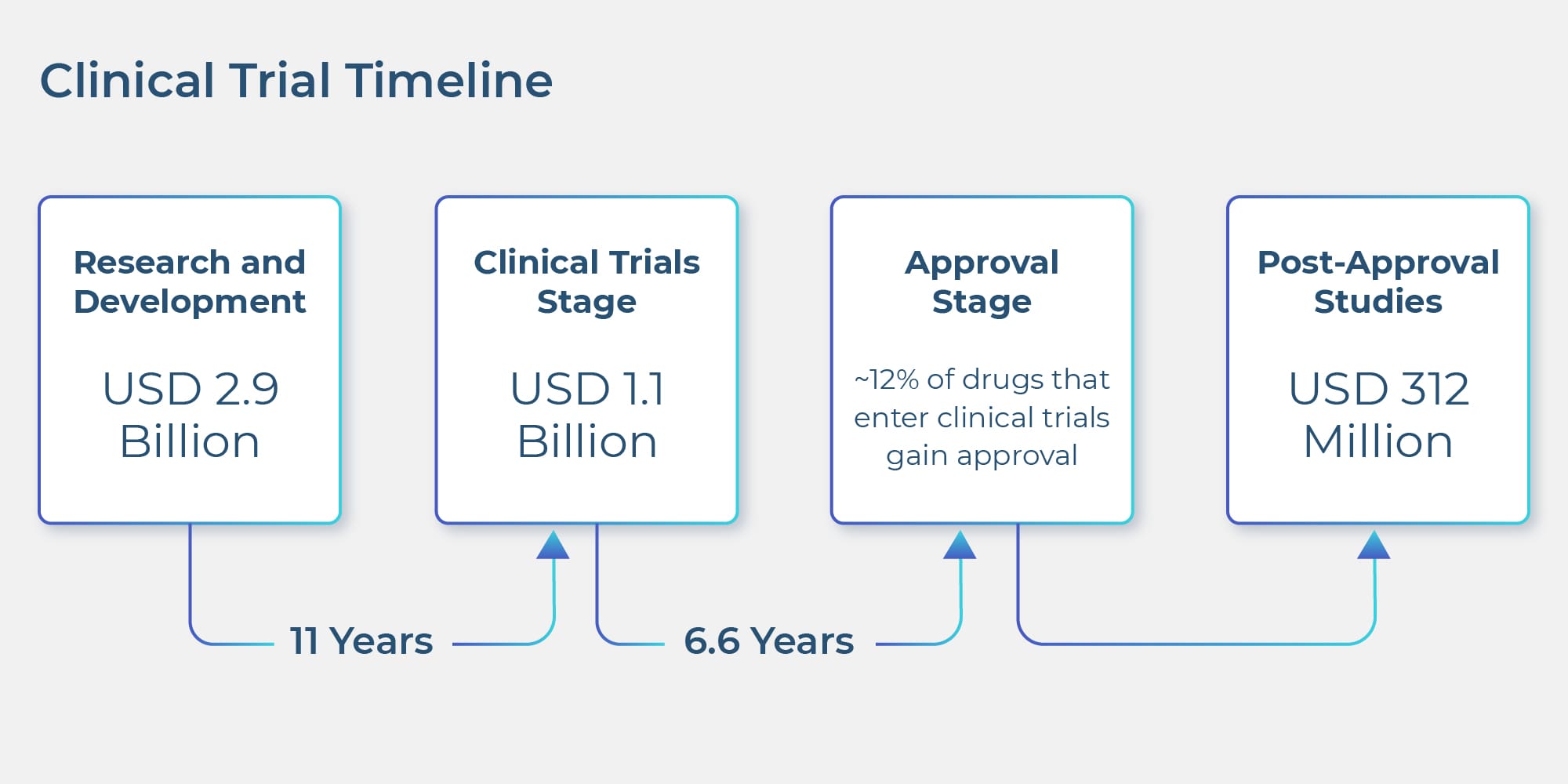
Biopharma companies are swamped with scientific and research data from a variety of sources like EMRs, lab images, research papers, ‘omics’ knowledge bases and the list goes on. Despite such humongous information silos, PhRMA reports that from drug discovery to FDA approval, the process of developing new medicines takes at least ten years. Out of which, only clinical trials consume six to seven years. Additionally, a recent industry survey revealed that less than 12 percent of drugs that enter clinical trials are approved.
The clinical trial market is enormous. Perhaps a reason why only clinical trials account for a staggering 40% of the entire pharma research budget. Despite the impressive size and heavy allocation of research budget, the clinical trial industry faces several challenges. The prime challenge being of cost. The image below depicts the clinical trial timeline along with the estimated budget for each stage.
A drug that cracks it from R&D to FDA approval can add hundreds of millions or billions to a pharma company’s assets whereas the cost of even just one failed drug candidate can set the business back by more investment. Look at the effects of failed clinical studies on some of the leading pharma companies like Novartis and Axovant; and you shall find it for yourself the aftermath of a failed clinical trial.
Why Do Clinical Trials Fail?
Clinical trials fail for a variety of reasons. Unsuccessful patient recruitment, mid-trail patient dropout, unintended and severe side effects, poor data collection methods to name a few. One thing common in all is the lack of skills and technologies to utilize the existing database effectively.
How Does Cognitive Search Transform Clinical Trials?
Artificial intelligence and machine learning algorithms of cognitive solutions index both structured and unstructured clinical trial data to give insights into the designs for future trials, making it low on costs and time. We have enlisted a few clinical trial use cases where AI-driven enterprise search tools can create a huge difference:
1. Efficient Patient Recruitment & Enrollment
One of the key reasons that leads to delays in clinical trials is patient recruitment and enrollment. Longer recruitment periods demand high costs and a great deal of time. Moreover, nearly 80% of clinical trials fail to meet enrollment timelines and due to further enrollment complications, one-third of Phase III clinical studies are terminated.
The idea behind implementing an AI-driven cognitive search solution is to enhance patient selection by mining, analysing and interpreting disparate data silos, including Electronic Health Records (EHRs), lab images and ‘omics’ knowledge bases like genomics, proteomics and metabolomics.
After selection comes enrollment wherein the shortlisted patients are required to tick the prerequisite checkboxes. The list of trial inclusion and exclusion criteria is often full of medical jargon. Not just cognitive search helps to decipher these jargon by leveraging NLP and text-analytics, but it also simplifies the enrollment process by verifying some of the preconditions automatically.
2. Improved Clinical Trial Designs
Biopharma companies have recognized that most of the clinical trials fail not because of the lack of drug efficacy, but because of unforced errors like inability to critically assess the existing designed trials or evaluate completed trials.
Only half of the drugs rejected by the FDA fail due to a lack of efficacy. Many of the remaining 50 percent are derailed by setbacks like failed attempts in abiding by the protocols, late form filings, non compliance with FDA’s feedback or a failed attempt in understanding the crucial parts of FDA’s review.
Powered by AI and ML, cognitive solutions have unparalleled potential to collect, organize and analyse the big data volumes, including scientific and research data from current & past clinical trials, electronic medical records (EMRs), post-market surveillance, and so on. By tapping into gathered data, research teams can identify meaningful patterns to improve future clinical trials.
3. Stellar Selection of Investigator Site
Identifying high-functioning investigator sites is of paramount importance for research teams as improper identification can lead to poor recruitment, affecting the overall operations of clinical trials. Site qualities such as resource availability, administrative processes, compliance with protocol requirements, understanding the disease and seasoned clinicians can influence both trial timelines and data quality and integrity.
Once the list of shortlisted sites is prepared, the most-suited site is then identified based on the key parameters involved in the protocol. By leveraging AI-driven tools, clinical researchers gather evidence to satisfy regulators about the compliance of the trial process. Thus, helping to decide on the site that would be able to meet the protocol requirements without compromising on the patient pool requirement and qualification and experience of investigators.
4. Patient Monitoring, Medication Adherence & Retention
Medication non-adherence may have adverse effects on the patient’s health. Recruiting new patients and disruption in the study timelines can turn out to be high costing. Therefore, clinical sponsors are desperately looking for technologies that could advance the practice of medication adherence, retaining more patients and reducing dropout rates.
AI algorithms of cognitive search platforms help monitor and manage patients by automating data capture, digitizing clinical assessments and sharing data across the system. Let’s say, an insight generated from biochemical experiments states that the most of the medication non-adherence happens because of the memory impairment of patients. In this scenario, cognitive tools can help with sending timely medication reminders. Such real-time communications can further help to retain patients and reduce dropout rates.
5. Generates Insights for Pharmacovigilance & Other Research Aspects
Clinical trials generate gigantic volumes of data and if not processed smartly, this data can hinder biopharma companies from having a comprehensive view of clinical trials; which can further risk drug safety, collaboration opportunities, designs for future trials, etc.
By leveraging AI and ML, cognitive search indexes both structured and unstructured data to generate intuitive and actionable insights. With the help of these insights, you can encourage self-learning, improved predictions and prescriptions, drug safety, and so on at your research center.
Want to Learn How Cognitive Technology Drives Clinical Trial Efficiencies?
The success mantra for a clinical trial is to create a patient-centric culture. SearchUnify is one such cognitive solution that leverages AI and ML to revolutionize clinical trials by attracting, monitoring, engaging and retaining committed patients throughout the research. It also gives insights into the past and current trials so that the future trials can be designed accordingly. For a hands-on experience of the technology, request a free demo today.